Smart packaging and traceable aerosol systems are no longer futuristic concepts reserved for pharmaceutical giants. With rising regulatory scrutiny, globalised supply chains and a surge in counterfeit health and beauty products, brands across personal care, healthcare, veterinary and specialist sectors are now exploring smart, serialised and digitally connected aerosols.
This playbook offers a step-by-step implementation roadmap — from scoping your first proof-of-concept to scaling a fully traceable, anti-counterfeit aerosol range with a specialist manufacturer.
Why Smart and Traceable Aerosols Are Moving from “Nice to Have” to “Must Have”
Three converging pressures are driving adoption:
1. Regulatory expectations
Sectors such as healthcare, medical devices and high-value OTC categories are under increased pressure to demonstrate traceability, batch integrity and anti-counterfeit protection throughout the product lifecycle.
2. Supply-chain risk
Global supply routes and third-party distributors create vulnerabilities around diversion, tampering and authenticity. Smart aerosols create a digital identity for each unit, enabling verification at any point.
3. Consumer confidence and brand protection
QR codes and digital product passports allow customers to authenticate products instantly. For premium skincare, clinical formulations and sensitive users, this strengthens brand credibility and reduces the risk of reputational harm.
Core Building Blocks – Sensors, Codes, Serialisation and Cloud
Smart aerosols sit at the intersection of physical packaging and digital infrastructure. Key components include:
Unique Identifiers
- GS1-compliant barcodes or QR codes
- Serial numbers etched, printed or embedded into components
- Hidden or dual-layer codes for anti-counterfeit use
Data Capture Technology
- NFC or RFID tags for proximity authentication
- Sensors for dose counting or actuation tracking
- Temperature or orientation sensors for specialist applications
Cloud and Verification Layer
- A secure database assigns meaning to each serial number
- Verification APIs allow retailers, healthcare providers and customers to confirm authenticity
- Audit logs enable investigation of suspicious activity or tampering
User-Facing Layer
- Simple QR scan for consumers
- Professional portals for pharmacists, clinics or distributors
- Integration with brand apps for adherence reminders, usage analytics or educational prompts
Together, these components create a closed-loop digital ecosystem, turning each can into a traceable, authenticated medical or consumer device.
Mapping Risk and Value: Which Products to Start With
Not all SKUs benefit equally. A smart aerosol programme should begin with products that have the highest exposure or greatest value uplift.
High-priority candidates
- Healthcare sprays where dose accuracy, traceability or sterility matter
- Premium skincare with risk of counterfeiting or adulteration
- Veterinary or agricultural aerosols prone to grey-market diversion
- Industrial products requiring controlled distribution or compliance auditing
Lower-priority candidates
- Low-value, high-volume household aerosols
- Products with minimal regulatory risk
- Non-differentiated commodity SKUs
Starting small ensures a focused, measurable rollout before committing to broader implementation.
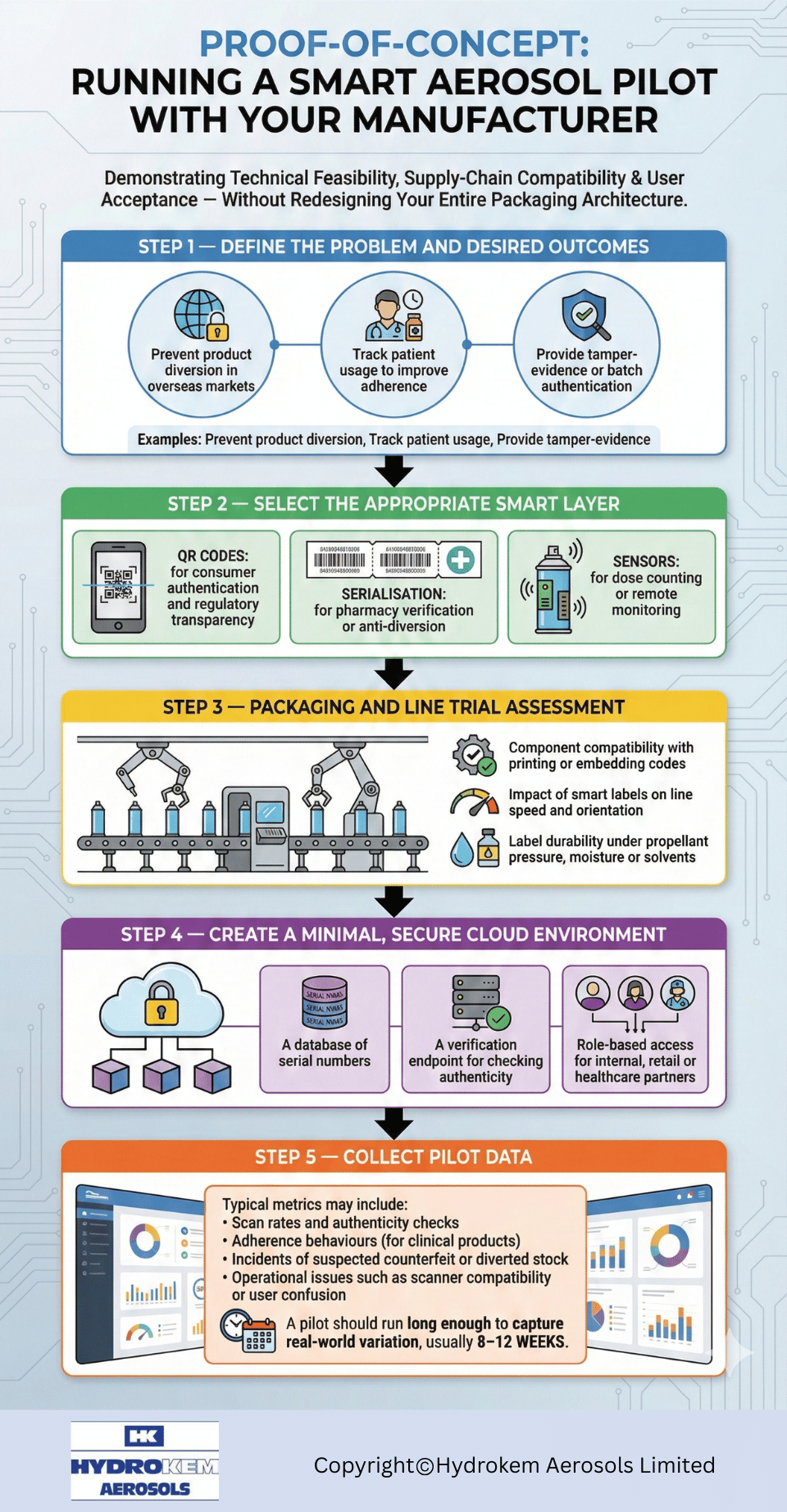
Proof-of-Concept: Running a Smart Aerosol Pilot with Your Manufacturer
A good proof-of-concept demonstrates technical feasibility, supply-chain compatibility and user acceptance — without redesigning your entire packaging architecture.
Step 1 — Define the problem and desired outcomes
Examples:
- Prevent product diversion in overseas markets
- Track patient usage to improve adherence
- Provide tamper-evidence or batch authentication
Step 2 — Select the appropriate smart layer
- QR codes for consumer authentication and regulatory transparency
- Serialisation for pharmacy verification or anti-diversion
- Sensors for dose counting or remote monitoring
Step 3 — Packaging and line trial assessment
A specialist manufacturer evaluates:
- Component compatibility with printing or embedding codes
- Impact of smart labels on line speed and orientation
- Label durability under propellant pressure, moisture or solvents
Step 4 — Create a minimal, secure cloud environment
This includes:
- A database of serial numbers
- A verification endpoint for checking authenticity
- Role-based access for internal, retail or healthcare partners
Step 5 — Collect pilot data
Typical metrics may include:
- Scan rates and authenticity checks
- Adherence behaviours (for clinical products)
- Incidents of suspected counterfeit or diverted stock
- Operational issues such as scanner compatibility or user confusion
A pilot should run long enough to capture real-world variation, usually 8–12 weeks.
Data, Privacy and Ownership – Questions to Answer Upfront
Who owns the scan data?
Ownership of scan data must be agreed contractually at the start of any smart aerosol programme, because different parties—brand, manufacturer, distributor or cloud provider—may all have a stake in how the data is generated, processed and used.
Detailed explanation:
When a smart or traceable aerosol includes QR codes, serial numbers, NFC tags or authentication markers, every scan creates a digital footprint. That footprint includes the time, location, user type, device type and verification result, and can be enormously valuable from a commercial, compliance and product-safety standpoint.
However, the question of data ownership is not automatic. In some implementations, brands assume complete ownership because the data relates to consumer behaviour and product integrity. In others, manufacturers retain operational data because they manage the serialisation, print application, or code management architecture. Distributors may have rights because scans often occur within their logistics networks, and cloud providers may assert rights over anonymised or aggregated datasets generated on their platform.
To avoid disputes, the data ownership model needs to be spelled out explicitly during the scoping phase. This includes:
Who owns raw scan events versus aggregated analytics.
Whether third parties may access the data and under what conditions.
How data can be used for marketing, pharmacovigilance or regulatory reporting.
What happens to historical data if the brand switches manufacturers or cloud providers.
A clear ownership framework prevents future conflicts, protects intellectual property and ensures that the investment in smart packaging continues to deliver long-term strategic value.
How will user data be anonymised?
Smart aerosols can collect sensitive information, so anonymisation protocols must be built into the system from the start—especially in healthcare, skincare and paediatric categories where privacy expectations and regulatory standards are strict.
Detailed explanation:
Even when a user simply scans a QR code or authenticates a product, the resulting data can be classified as personal or behavioural information. It may include device identifiers, approximate location, time stamps or usage patterns. In beauty, healthcare and paediatric sectors, improper handling of such information can breach privacy laws, create ethical concerns or expose brands to reputational damage.
Effective anonymisation requires a multi-layered approach:
Removal of direct identifiers, such as IP addresses or device-specific markers, before data is stored.
Aggregation techniques, where data is grouped to avoid pinpointing individual users or vulnerable subgroups.
Randomisation or tokenisation, ensuring individual scan behaviours cannot be reconstructed.
Separation of operational and behavioural datasets, where logistics scans (by distributors or healthcare professionals) are stored differently from consumer scans.
Some jurisdictions—especially for medical and paediatric products—require additional safeguards such as differential privacy or strict access controls. For healthcare aerosols, anonymisation must also align with pharmacovigilance expectations, where de-identified usage data may support adherence programmes or product-safety monitoring.
An effective anonymisation strategy protects users, meets regulatory standards, and builds trust that smart aerosols enhance safety without compromising privacy.
What retention rules apply?
Retention periods for traceability data depend heavily on sector classification, with healthcare, medical device and regulated OTC products often requiring long-term records that support audits, recalls and compliance investigations.
Detailed explanation:
The question of how long you keep smart-pack data is closely tied to the regulatory environment in which your aerosol operates. For low-risk consumer products, retention might simply follow standard corporate policies or marketing analytics cycles. But for healthcare, clinical, veterinary, or medical-device aerosols, retention rules can be significantly more stringent.
Key considerations include:
Regulatory retention requirements, which may mandate keeping traceability, batch and authentication records for 5, 10 or even more years, depending on product type and jurisdiction.
Recall readiness, where historical serialisation logs can be critical to identifying affected units and demonstrating due diligence.
Audit and inspection expectations, especially for medical-grade aerosols, where regulators expect robust documentation that shows product integrity across its lifecycle.
Litigation and liability protection, where retaining historical authentication data helps defend against claims of counterfeit or diverted stock.
Because smart aerosols generate far richer traceability data than traditional packaging, organisations must decide what is retained, where it is stored, and who can retrieve it. Choosing a retention strategy early prevents fragmentation and ensures compliance with varying sectoral or geographical rules.
How will data integrate with your existing systems?
Smart aerosol data must integrate cleanly with systems like ERP, CRM, quality management, pharmacovigilance and marketing analytics — otherwise its value is lost and operational friction increases.
Detailed explanation:
One of the biggest hidden challenges in smart packaging is ensuring that newly generated data does not become another silo. Every scan, authentication event or usage pattern is potentially valuable, but only when it can be connected to the systems your business already uses.
Integration typically includes:
ERP and supply-chain systems, where serialisation data supports batch traceability, stock accuracy and anti-diversion monitoring.
Quality Management Systems (QMS), where authentication and failure logs help detect emerging defects or tampering risks.
Pharmacovigilance or clinical-safety systems (for applicable products), where usage patterns or abnormal scan behaviour could support safety monitoring.
CRM and marketing platforms, where anonymised consumer scans feed loyalty programmes, product education or aftersales journeys.
Data warehousing or analytics tools, which bring together operational and behavioural datasets to support forecasting, compliance and commercial decisions.
For integration to work, brands must define:
Data formats and transfer protocols (API, EDI, secure batch export).
Frequency of data synchronisation.
Validation and clean-up processes to ensure data accuracy.
Governance around who can access integrated datasets.
Without proper integration, smart aerosol data remains isolated and cannot deliver full value to your business or supply chain.
What are your cyber-security obligations?
Smart aerosol systems rely on serialisation, cloud databases and verification tools — so poorly secured APIs, weak authentication or exposed endpoints can compromise the entire programme.
Detailed explanation:
Smart packaging elevates an aerosol from a static object to a connected digital asset. This creates new cyber-security responsibilities. Attackers may attempt to spoof serial numbers, inject false authentication results, scrape tracking endpoints or exploit weak API protections. Any vulnerability in the system undermines the credibility of your anti-counterfeit and traceability programme.
Core considerations include:
API security: All data exchanges must use encrypted channels, rate limiting and authentication tokens to prevent scraping or brute-force attacks.
Permissions and access control: Only approved partners (e.g., distributors, pharmacists, authorised retailers) should be able to validate codes at a deeper level.
Tamper-resistant code generation: Serial numbers must follow secure, non-predictable algorithms to prevent counterfeiting.
Cloud platform resilience: Redundancy, intrusion detection, incident response and monitoring must all be in place.
Protection against data manipulation: Any attempt to alter or overwrite authentication logs must be impossible or detectable through audit trails.
Supply-chain cyber hygiene: Third-party partners must meet minimum standards to prevent weak links.
If cyber-security is treated as an afterthought, it often becomes the costliest part of the programme to fix. Establishing a secure architecture and governance model early prevents re-engineering later and preserves the integrity of your serialisation and traceability investment.
Rolling Out at Scale – Manufacturing, Packaging and Logistics Integration
Scaling smart aerosols requires aligning your entire supply chain.
Manufacturing integration
- Code printing or serial application must be validated
- QC must incorporate verification of readability and uniqueness
- Line speeds may need adjustment for certain smart technologies
Component supply chain
- Ensure suppliers can meet tolerances for sensors, tags or variable-data labels
- Validate adhesives, inks or materials under pressurised conditions
Warehouse and distribution alignment
- Distributors must be equipped to scan codes and share data
- Overseas partners may require training to reduce mishandling or false alarms
- Anti-diversion controls should be tested across multiple markets
Retail and pharmacy support
- Provide training and digital assets to ensure the authentication process is easy
- Ensure QR or code placement is visible but tamper-resistant
- For clinical settings, ensure devices meet relevant documentation standards
Smart packaging only works when every touchpoint participates — from filling line to end user.
Measuring Success – Compliance, Counterfeit Reduction and User Insight
Successful smart aerosol programmes deliver quantifiable value, not just technological novelty.
Key performance indicators include:
Compliance and audit performance
- Reduction in incomplete or ambiguous batch records
- Improved traceability documentation for inspections
- Faster root-cause investigation due to digital audit trails
Counterfeit and diversion mitigation
- Number of authentication scans per geography
- Rate of failed or suspicious scans
- Reduction in unauthorised distributor activity
Consumer and clinical behaviour insight
- Real usage patterns from scan data or sensor output
- Improved adherence for medical or skincare protocols
- Customer support reduction due to clearer product information
Operational efficiencies
- Fewer product returns due to authenticity disputes
- Lower cost of handling investigations
- More accurate forecasting and stock allocation
When implemented correctly, smart aerosols become self-improving systems, generating data that continuously strengthens decision-making, quality, compliance and brand value.
Smart, traceable and anti-counterfeit aerosols transform packaging from a passive container into an active participant in safety, compliance and user experience. Whether your priority is authenticating premium skincare, preventing medical diversion, tracking patient adherence or protecting veterinary supply chains, the key to success lies in a structured, phased implementation guided by technical and regulatory specialists.
As brands look to future-proof their ranges and stand out in competitive markets, smart aerosols offer a strategic advantage that blends technology, compliance and consumer trust — and those who invest early will shape the new standards of product integrity.








